By Louise Vercruysse, Joost Sleiderink, Aron Ortega, Esther van Hoof & Pablo Van Neste
Join our event The phosphorus crisis: an alternative look from the forest , online, on the 12th of May!
Phosphorus, Phosphate, P-levels are probably terms you have heard of in chemistry, biology or geology class. It is one of the essential minerals needed to build life, acting as a chemical backbone to create structures. Your DNA, RNA, membranes, metabolism and bones consist of phosphorus and every day you need to replenish it. Not only is it vital for human life, it is also critical to the plants and animals that feed us. Phosphorus is a special mineral, has a sticky structure and was the first mineral to be separated in modern science. We might not acknowledge it enough, but it is one of the most important resources for our survival. But in gaining more control over phosphorus as a resource, we are losing it even more rapidly. There seems to be a paradox.
What is, then, the phosphorus paradox?
Phosphorus is a finite resource, unequally distributed throughout the world. Currently the biggest phosphate mines are located in China, in Morocco and the West-Sahara and in the United States. The demand for phosphorus is expected to rise exponentially because of the growing world population, while the current phosphorus reserves are likely to be depleted in 80 years. Currently, phosphate levels in the soils in EU and North-America are relatively high, due to decades of application and consumption of the raw material has been on the decline due to regulations and prices. In fact, little application is needed to keep yields on levels where they are for decades to come. In Asia the demand is still rising due to the increasing popularity of meat (livestock systems require high phosphorus inputs). Currently, 90 percent of phosphorus is mined to produce fertilizers used in industrial agriculture. About 1/10th ends up in our food, and then in our solid waste, which goes down the sewage pipe, is processed into sewage sludge and then burned to be made into concrete and asphalt. The part of the phosphate in fertilizers that is not directly taken up by plants, binds in the soil or is lost to erosion and is then made unavailable. This means that a lot of phosphorus fertilizer is needed to supply crops, because a great deal of it will be made unavailable upon contact with soil particles. When too much fertilizer is used, however, all the binding sites can become occupied, which causes the excess phosphates to wash out into waterways. Too much phosphorus in waterways causes eutrophication, an excessive richness of nutrients, which leads to the depletion of oxygen in ocean waters, making it impossible for local fauna and flora to thrive. While every organism needs enough phosphorus to survive, an excess of the nutrient creates dead zones.
A short history of phosphorus
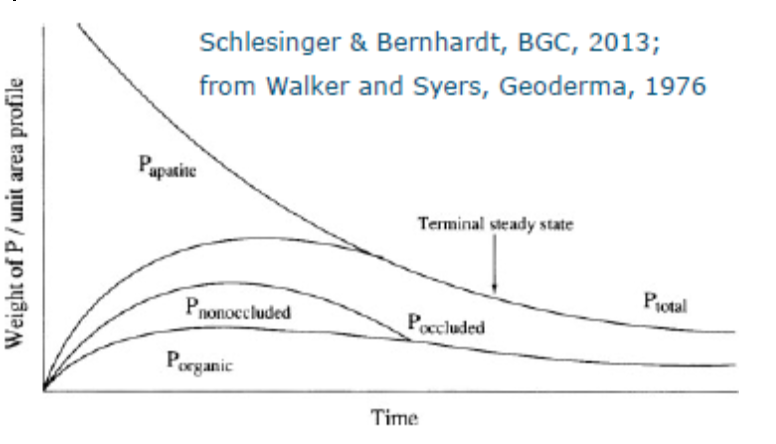
Historically natural phosphate levels determined soil fertility and productivity. Phosphate in soils originates from the weathering of phosphate rich minerals such as apatite. Over time, the weathering of primary minerals results in a decreasing total amount of phosphate in (natural) systems, which, next to the composition of minerals, also contributes to global differences in natural fertility with soil age. The natural phosphate cycle is centered around vegetation, producing litter and fostering other organic inputs, which contribute to the natural cycle of (organic) phosphorus. As from the early history of hunter-gatherers onwards, humans have used techniques to increase this natural cycling of nutrients for example by using fire to increase the input of organic matter. Later, the use of manure and human excreta also started to be used on agricultural fields. The increase in fertility was then a result of the expansion of the area from which phosphorus was collected for crop use. The use of human waste however diminished with the start of the Industrial Revolution and the growth of city populations far from rural areas, which largely affected global phosphorus cycles, because sanitation became more focused on municipal disposal than on reuse. The next major change in phosphorus use in agriculture came about in the middle of the 19th century when Justus von Liebig confirmed that fertilization due to organic matter was due to the inorganic nutrients that it released, reducing the importance of fertilizer only to its compounds. This had a large effect on Western agriculture especially. Crushed bones and guano (bird and bat droppings off the coast of Peru) were imported to the West, which contained very high levels of phosphorus, nitrogen and potassium, but soon became scarce. It was clear that other sources were needed. After World War II phosphate rock mining (fertilizer) increased tremendously during the Green Revolution, which allowed for large human population growth, but also further decoupled supply, demand and waste of phosphor in the natural cycles. Many farmers were told that an increase in yield went hand in hand with the addition of fertilizers and mining projects were stimulated even further.
The site of secondary mining of Phosphate rock in Nauru, 2007. Photo: Lorrie Graham CC BY 2.0
Phosphate mining
Phosphate mining is one of the biggest threats to our environment. Not only is it polluting lakes and rivers all over this planet, but the Environmental Protection Agency (EPA) has publicly stated that the way they mine and produce the fertilizer is one of the most toxic industries in the United States. Environmental risks related to phosphate mining are amongst others: contamination of surface water, the release of radioactive compounds such as Uranium and contaminating soil with heavy metals such as Cadmium. It is, however, important to note that the phosphorus footprint of a country varies, largely due to the amount of meat consumption. The average per capita human dietary phosphorus footprint has increased 38% globally since 1970. There are important differences in the phosphorus footprint of countries, where countries with high Human Development Index (HDI) values typically consume larger amounts of animal products and have significantly higher phosphorus footprints than lower HDI countries. Although still not as high as the phosphorus footprints of the USA or Argentina, emerging economies have shown rapid phosphorus footprint increases over time (e.g., China’s phosphorus footprint has increased by 400% since 1970).
Unequal distribution
There is an additional perspective to be looked at. There is a general distinction between soils in temperate and tropical regions. As mentioned above, phosphate originates from the weathering of rocks and minerals, but so do the minerals that bind strongly to phosphate and make it unavailable for plant uptake. Hence, the more weathered a soil is, the more of these phosphate binding minerals it contains. Soil weathering is a function of both climate and time. Older soils and soils that receive more rainfall and experience higher temperature will thus generally have a higher capacity to bind phosphate.
During the last couple of ice ages, the encroaching ice caps functioned as massive geological scrapers, shaving off the top soil in large parts of the continental northern hemisphere. When the ice caps melted and receded again they revealed a fresh, young topsoil. The process of soil formation had to start all over.
The ice caps, however, never reached the regions we now classify as (sub-)tropical. These soil have undergone orders of magnitude longer timescales of weathering, resulting in highly P binding soils. These soil therefore have an inherently low fertility and P fertilization is also a lot less efficient which leads to problems with agricultural productivity.
Furthermore, tropical soils have experienced high amounts of rainfall which has dissolved and carried away most of the soluble minerals and tropical soils generally have a vegetation layer that is relatively thin and nutrient poor. The soil horizons are shallow, and often not very clearly distinguished. Agricultural practises often cause additional deterioration of the already poor and shallow soils, causing the soil to not hold onto water well with a high percentage of surface runoff. Temperate soils accumulate deep vegetation and are clearly banded into the A, B, and C horizons. The soils tend to be darker because the dark minerals have not been dissolved. The ground has a high capacity for water absorption and is nutrient rich.
The tropical regions on this planet also contain the largest fraction of formerly colonized countries, where farmers to this day do not have the same access to resources as farmers in the “West”. Besides that, in the European Union, the US and China amongst others, farmers receive subsidies for artificial fertilizers, which many developing countries do not. On average, fertilizers cost 2 to 6 times as much in Africa than in Europe and Northern America and Asia. P fertilizer is thus mainly funnelled into the more temperate regions, even though mining occurs mainly in the more tropical regions. This stacking of geological and socio-economic inequalities contributes and exacerbates the problems with hunger in these regions.
How can we avoid running out of phosphorus?
 We have to use phosphorus more efficiently in agriculture. This can be done for the greatest part by improving its acquisition. Let’s dive into some deep science. Phosphate is an immobile nutrient. Consequently, roots have to grow in search of phosphorus instead of having the phosphate come to them. Certain plant or crop species have developed adaptations to achieve this. A prime example of this in an agriculturally relevant plant are the cluster roots of white lupin, although other crops like macadamia perform the same trick. Cluster roots are bottlebrush-like short lived fine roots, capable of fully exploring patches of soil in search of phosphorus when these crops experience phosphorus limited conditions (see figure below). These cluster roots also secrete large amounts of organic acids which can dissolve the strongly bound phosphate from the mineral soil particles so the crop can take it up. An added benefit of these organic acids is also that they increase microbial activity and thereby P availability. Growing more crops with inherent adaptation to P limited environments or intercropping or rotating crops which have high P demands with crops that have these special adaptations to make P available could greatly increase P efficiency.
We have to use phosphorus more efficiently in agriculture. This can be done for the greatest part by improving its acquisition. Let’s dive into some deep science. Phosphate is an immobile nutrient. Consequently, roots have to grow in search of phosphorus instead of having the phosphate come to them. Certain plant or crop species have developed adaptations to achieve this. A prime example of this in an agriculturally relevant plant are the cluster roots of white lupin, although other crops like macadamia perform the same trick. Cluster roots are bottlebrush-like short lived fine roots, capable of fully exploring patches of soil in search of phosphorus when these crops experience phosphorus limited conditions (see figure below). These cluster roots also secrete large amounts of organic acids which can dissolve the strongly bound phosphate from the mineral soil particles so the crop can take it up. An added benefit of these organic acids is also that they increase microbial activity and thereby P availability. Growing more crops with inherent adaptation to P limited environments or intercropping or rotating crops which have high P demands with crops that have these special adaptations to make P available could greatly increase P efficiency.
However, crops that don’t have these adaptations are not at a loss, since P acquisition could also be improved through breeding. Other root traits that could be bred for to improve P acquisition are to increase the amount of roots in the topsoil, where most P is found, and increase lateral branching and root hair formation. Also, roots could be bred to favor elongation over expansion and could decrease the amount of living cells to the minimum requirement for root functioning. All to increase the capacity of a crop to explore the soil to its best ability for the lowest metabolic costs. Furthermore, plants that don’t have cluster roots can still secrete the earlier mentioned organic acids and other compounds to aid P uptake, which has been found in for example rapeseed and could be improved through breeding. But also above-ground we can find inventive ways to reduce P losses. Plants can for example retrieve parts of the phosphorus from their old leaves before they drop.
But if we zoom out for a minute from the individual crop to the ecosystem level and look at the natural ecosystems agriculture is embedded in, we can ask ourselves the question: “How is it that the natural systems on this planet, even the most productive ones, do not need fertilization? Where do they get their phosphorus from?
The first lesson we can draw observing natural systems is nutrient recycling. In nature there is no large export of nutrients off the fields and forests and what is taken up by plants or eaten by animals is returned and recycled. In agricultural systems, a large part of the phosphorus, mined at high environmental costs, ends up in our excrements and thereafter in the sewage system not to be returned to the field and therefore effectively lost. Recycling our humanure again as in the old days is the only way to close the agricultural phosphorus cycle. Incentivised by higher fertilizer prices and carbon taxes this could be achieved. Although most of the recoverable nutrients are currently lost, centralized municipal collection facilities offer a means to recycle large quantities of phosphorus with relatively little effort. However, in our current system the infrastructure does not exist to do this on a large scale and the price of synthetic fertilizer is still too low in countries that consume the most of it.
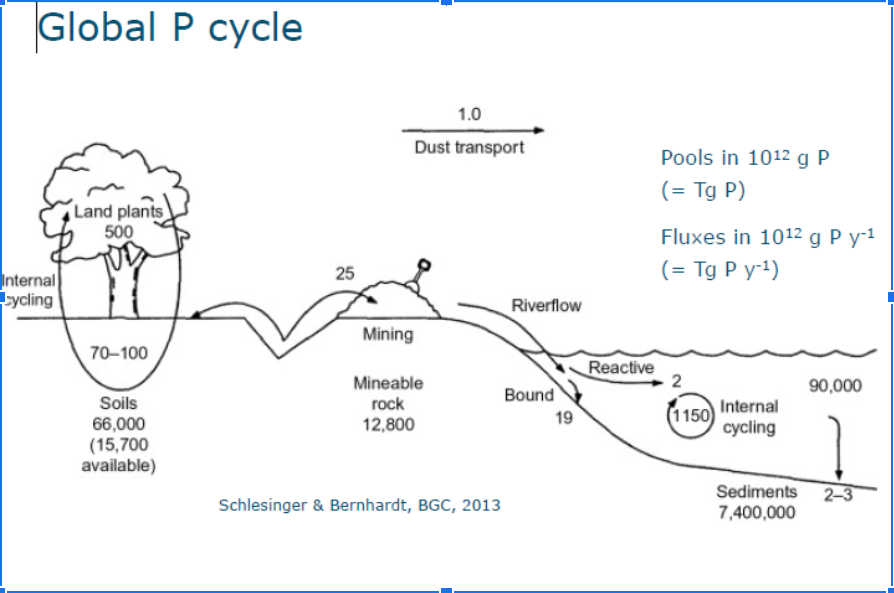
P cycle (2013)
The second lesson is that in nature there exists complementarity through diversity. Plants in natural systems have co-evolved to avoid competition for nutrients by having different but complementary rooting strategies. Some root shallowly, some root deeply, some exude organic acids to dissolve P, others fix nitrogen from the air, and still others draw up moisture from the groundwater to the topsoil. Together these combinations of plants make sure that nutrients are put into the system, are available for uptake and are not lost due to leaching or erosion so that productivity can be maintained.
Thirdly we can observe that natural systems are dominated by perennial plants. Perennial plants are those that grow for multiple years in a row. These plants feed the soil microbial community all year round, unlike annuals which grow only for a couple of months before decaying. They also foster higher levels of soil organic matter (SOM). Due to the combination of higher levels of SOM and microbial abundance, perennials have been found to maintain higher levels of P in organic form, which is more easily available compared to inorganic P, which binds strongly to the soil. P availability is also driven by pH and chemical equilibria which stabilizes in perennial systems.
Lastly, natural systems lack the periodic soil disturbance characteristic of conventional agriculture. Plowing reduces microbial abundance and diversity. In the case of phosphorus, the mycorrhizal fungi are the most important class of microbes that are reduced in number by tillage. More than 80% of land plants have evolved symbiosis with these fungi which greatly increase soil exploration for phosphorus and exchange it with its host plant in exchange for sugars made during photosynthesis. However, in agricultural settings, these fungi are periodically destroyed before reaching their full potential. No-tillage management, inherent to perennials, will thus be necessary to increase agricultural P efficiency.
Now that we learned from the strategies of natural ecosystems to achieve P self-sufficiency, we can inform the design of our agricultural systems based on these principles. Niek Pepels is a student of agro-ecology at Wageningen University and has specialized in phosphorus dynamics and the role of mycorrhizal symbiosis in agroforestry systems and will give a talk about this followed by a Q and A session. This 12th of May, from 19:30-21:00.
Sources
Ashley, K., Cordell, D., & Mavinic, D. (2011). A brief history of phosphorus: from the philosopher’s stone to nutrient recovery and reuse. Chemosphere, 84(6), 737-746.
Crews, T. E., & Brookes, P. C. (2014). Changes in soil phosphorus forms through time in perennial versus annual agroecosystems. Agriculture, ecosystems & environment, 184, 168-181.
Cordell, D., Drangert, J. O., & White, S. (2009). The story of phosphorus: global food security and food for thought. Global environmental change, 19(2), 292-305.
Hoffland, E. (1992). Quantitative evaluation of the role of organic acid exudation in the mobilization of rock phosphate by rape. Plant and Soil, 140(2), 279-289.
Lynch, J. P. (2011). Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant physiology, 156(3), 1041-1049.
Neumann, G., & Martinoia, E. (2002). Cluster roots–an underground adaptation for survival in extreme environments. Trends in plant science, 7(4), 162-167.
Plaxton, W. C., & Carswell, M. C. (1999). Metabolic aspects of the phosphate starvation response in plants. Plant responses to environmental stresses: from phytohormones to genome reorganization. Marcel Dekker, New York, 349-372.
Vance, C. P., Uhde‐Stone, C., & Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New phytologist, 157(3), 423-447.
Picasso, V. D., Brummer, E. C., Liebman, M., Dixon, P. M., & Wilsey, B. J. (2011). Diverse perennial crop mixtures sustain higher productivity over time based on ecological complementarity. Renewable Agriculture and Food Systems, 26(4), 317-327.
Porder, S., Vitousek, P. M., Chadwick, O. A., Chamberlain, C. P., & Hilley, G. E. (2007). Uplift, erosion, and phosphorus limitation in terrestrial ecosystems. Ecosystems, 10(1), 159-171.
Sanchez, P. A. (2002). Soil fertility and hunger in Africa. Science, 295(5562), 2019-2020.
Zhao, X., Dong, Q., Ni, S., He, X., Yue, H., Tao, L., … & Shen, J. (2019). Rhizosphere processes and nutrient management for improving nutrient-use efficiency in macadamia production. HortScience, 54(4), 603-608.
https://web.mit.edu/12.000/www/m2016/finalwebsite/solutions/phosphorus.html
https://www.frontiersin.org/articles/10.3389/fnut.2016.00035/full
Root figure from https://www.botany.one/2016/10/phosphoenolpyruvate-carboxylase-activation-reversible-phosphorylation-white-lupin-cluster-roots/